On February 21, 2020 the U.S. Food and Drug Administration (FDA) accepted BioMarin Pharmaceutical’s submission for Valrox when it agreed to undertake a priority review of the company’s biologics license application for the drug. Valrox is the trade name for valoctocogene roxaparvovec, a gene therapy for adults with hemophilia A. The target date for their decision is August 21, 2020, and a similar request is also before the European Medicines Agency.
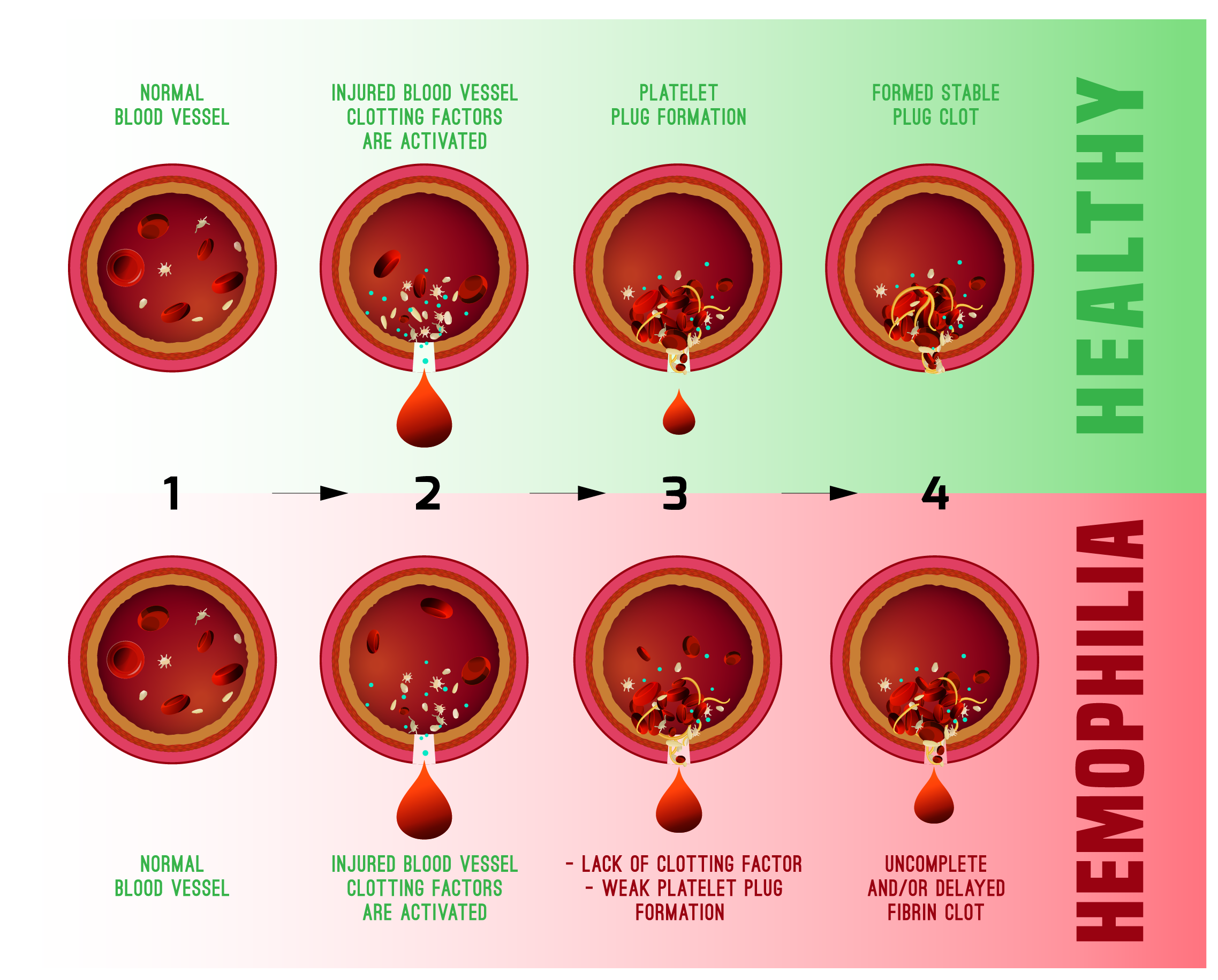
Hemophilia A is a genetic disease caused by missing or faulty factor VIII. This most common type of hemophilia occurs more frequently in males with an estimated incidence of one in 4,000-5,000 male births. About two-thirds of patients have a mutation that came from their parents, while others have a spontaneous, or not inherited, mutation.
More than 400,000 people are estimated to have this inheritable blood disorder. Hemophilia A is the most common form, and it is estimated that approximately 20,000 affected people are in the US. BioMarin puts the current cost of blood clotting treatments at $25 million per patient over a lifetime. Many hemophilia A patients still have bleeding episodes despite preventive treatment.
Should it be approved, BioMarin is considering pricing Valrox at $2 million to $3 million for the single treatment. At that cost, it would make Valrox the world’s most expensive one-time therapy. The company’s position is that this first gene therapy could save healthcare systems more than $20 million over a typical patient’s lifetime.
BioMarin is open to alternative payment structures for Valrox, such as a pay-for-performance structure. However, as Chairman and Chief Executive Officer Jean-Jacques Bienaime noted at the JP Morgan Healthcare Conference in January 2020, health insurers appeared to be comfortable with the proposed $2-3 million price range for Valrox’s single-use treatment. Most insurers indicated in discussions with the company that they expect to make single, upfront payments.
Clinical trial results published in the New England Journal of Medicine in February show that a single infusion of Valrox at its higher doses led to a greater than 90% reduction in mean annualized bleeding rates, including in target joints, as well as a corresponding decrease in the need for preventative use of factor VIII treatments (the current standard of care). This clinical trial is an open label Phase 1 / 2 trial. A global Phase 3 study is now underway.
Trial data showed results of seven patients given the highest gene therapy dose. They had a mean annualized bleed rate of 16.3 and a mean of 136.7 factor VIII infusions a year. Three years post treatment, their annualized bleed rate had declined to a mean of 0.7 and the number of annual factor VIII infusions had declined to a mean of 5.5, a 96% change. In another group (6 people), the mean annualized bleed rate fell from 12.2 per year in the year prior to the study to 1.2 at the end of the second year, representing a 92% decrease.
Although the therapy’s efficacy and safety data were positive, there are some concerns over the duration of response, with some physicians suggesting that it will begin to lose clinical benefit if the Factor VIII levels do not hit a plateau. Some physicians describe the numbers as indicating a conversion of severe hemophilia patients into mild hemophilia patients as opposed to a complete cure. Currently there is no evidence that the drug produces a steady, long-term response. The FDA will be watching closely for durability of response and the possibility of long-term malignancy when it comes to approval.
Experts are divided on exact factor VIII benchmarks needed for success in clinical practice due to discrepancies between the levels necessary to change a patient’s quality of life and the high standards expected of hemophilia treatments in development. Experts want to see valoctocogene roxaparvovec (valrox) achieve sustained factor VIII levels of approximately 55%. However, investigators have indicated they would be satisfied with median factor VIII levels sustained in the 30–50% range. One noted a more modest level of 20–30%. Patients with these lower levels would definitely be protected from spontaneous bleeds, but would need to be treated for trauma or surgeries, as one investigators explained. In addition, investigators note that if levels drop below 10% the clinical benefit will be lost.
Please contact your Summit Re representative as soon as you become aware of a request for Valrox or any other gene therapy. Our Summit ReSources managed care staff is available to answer any questions or concerns you may have. We will continue to investigate and provide information to our clients as this field of medicine continues to evolve.
Article by Ginny Fisher, RN, BSN, CCM, Managed Care Specialist. For more information about how this may affect your plan, please contact your Summit ReSources care specialist. The following sources were used as reference material for this article:
Copyright © 2020 Summit Reinsurance Services, Inc. All rights reserved. May not be reproduced without written permission.